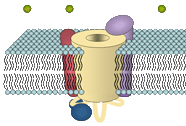
Cell culture & model systems

For research on skeletal muscle EC coupling two systems are used in the lab. On the one hand we routinely culture an immortalized murine muscle cell line (GLT) deficient for the α1S subunit of CaV1.1 (muscular dysgenesis; mdg). This cell line is used for expression studies of α1 chimeras and point mutants. For investigating the role of the β1 subunit in skeletal muscle EC coupling we use freshly dissociated myotubes from a zebrafish strain deficient for the β1a subunit (relaxedts25). This system enables us to work with highly differentiated myofibers. For transient expression studies of selected Ca2+ channel constructs we routinely perform different transfection methods including lipofection and electroporation.
Molecular Biology
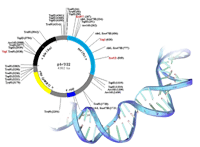
In order to explore structure-function relationships of different Ca2+ channel subunits we selectively exchange channel fragments with the corresponding elements of a related channel isoform ('chimeras') or we generate single amino acid changes ('point mutants'). cDNA constructs coding for GFP-tagged recombined channel subunits are inserted into mammalian expression vectors (for transfection) or into polyadenylating transcription vectors (for cRNA injection) to be subsequently expressed in the corresponding knock-out cells. Our molecular biology lab has a long standing experience in the techniques of molecular cloning of different Ca2+ channel subunits ranging from phage-library screening techniques, PCR cloning strategies, Northern and Southern blotting, whole-mount in-situ hybridisation and site directed mutagenesis to different PCR-techniques (including fusion PCR, RT-PCR and real-time) as well as to genomic analysis methods like RFLP or genome walking.
Intracellular calcium signalling
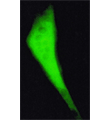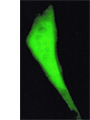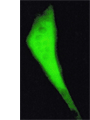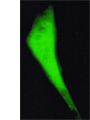
Changes in intracellular Ca2+ level in response to extracellular electrical stimuli is measured in field stimulation experiments. Living cells are thereby loaded with Ca2+ indicators Fluo-4 or Indo-1. By eliciting action potentials the release of Ca2+ from intracellular stores is induced. The resulting change in intracellular Ca2+ levels can be recorded via changes in cellular fluorescence and is used to analyze action-potential-induced Ca2+ transients.
Electrophysiology
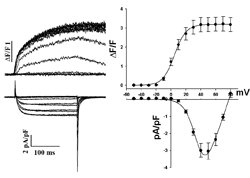
To characterize the biophysical properties of Ca2+ channels in EC coupling we apply combined measurements of electrophysiology and fluorescence life cell imaging. The whole-cell patch clamp technique is used to apply membrane depolarizations to different test potentials to a single muscle cell. Thereby the Ca2+ influx integrated over the cell surface membrane is measured. Loading of the myotubes with a Ca2+ sensitive dye allows a simultaneous fluorometric measurement of changes in the intracellular Ca2+ level, almost exclusively produced by Ca2+ release from the sarcoplasmic Ca2+ stores through the ryanodine receptor.
Zebrafish zygote injection

To test for the influence of different heterologously expressed Ca2+ channel subunit constructs on the intact muscle in-vivo, we inject in-vitro synthetized RNA coding for these constructs into zygotes of zebrafish. The muscle performance is tested in the developing embryo. Transient knock-down of Ca2+ channel subunits can be performed by injection of morpholino antisense oligos, and by injection of DNA a germline transmission of the desired construct can be archieved to generate stable expressing transgenic zebrafish lines.
Digital imaging
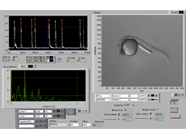
In close cooperation with the laboratory of Thorsten Schwerte we are analyzing zebrafish larval motility. Video sequences of zebrafish larvae are captured and converted to curves, which can be analyzed for frequency, intensity and shape of single twitches. This method allows us to study implications of EC coupling changes on the intact organism.