|
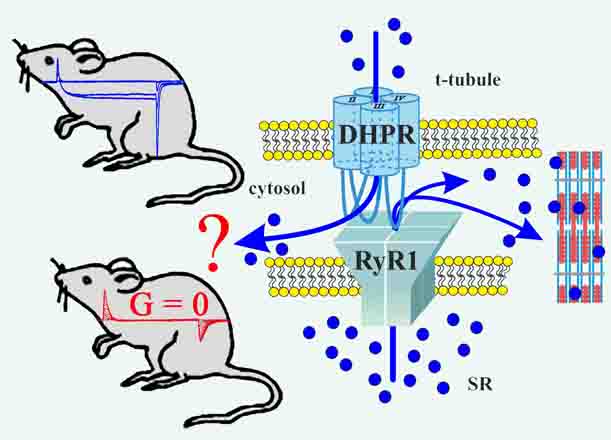
2017: A KI mouse model with a non-Ca2+-conducting skeletal muscle DHPR (ncDHPR) was created to explore the specific role of the DHPR-mediated Ca2+ current in skeletal muscle via a plethora of approaches. Surprisingly, these experiments did not point to any differences between ncDHPR mice and their wild-type (wt) littermates, irrespective of whether mice were young or aged. Overall, our results strongly support the hypothesis that the Ca2+ influx through the mammalian skeletal muscle DHPR is irrelevant for muscle performance and consequently can be considered as an evolutionary remnant (vestigial) of the phylogenetic stages of early chordates, where DHPR Ca2+ influx was the exclusive trigger for RyR activation in skeletal-muscle EC coupling. (Cooperation with Melzer lab). Dayal et al., 2017, Nature Communications |
|
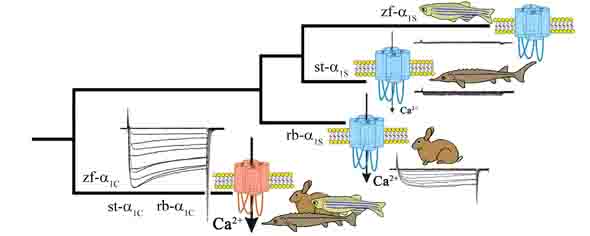
2017: Biophysical and phylogenetic analyses of the skeletal muscle DHPR of the early ray-finned fish species sterlet (Acipenser ruthenus) support the hypothesis that DHPR Ca2+ conductivity “fades out” during vertebrate evolution due to the lack of evolutionary pressure on this presumably vestigial current. Ca2+ conductivity through the sterlet skeletal muscle DHPR (which is more evolved compared to rabbit) is significantly reduced, but in contrast to zebrafish it still Ca2+ conducting. However, the final “turn off” of DHPR Ca2+-conductivity did not take place before the stage of euteleost fishes and was preceded by the teleost-specific 3rd round of whole genome duplication, Ts3R. Schrötter / Dayal & Grabner, 2017, Cell Calcium |
|
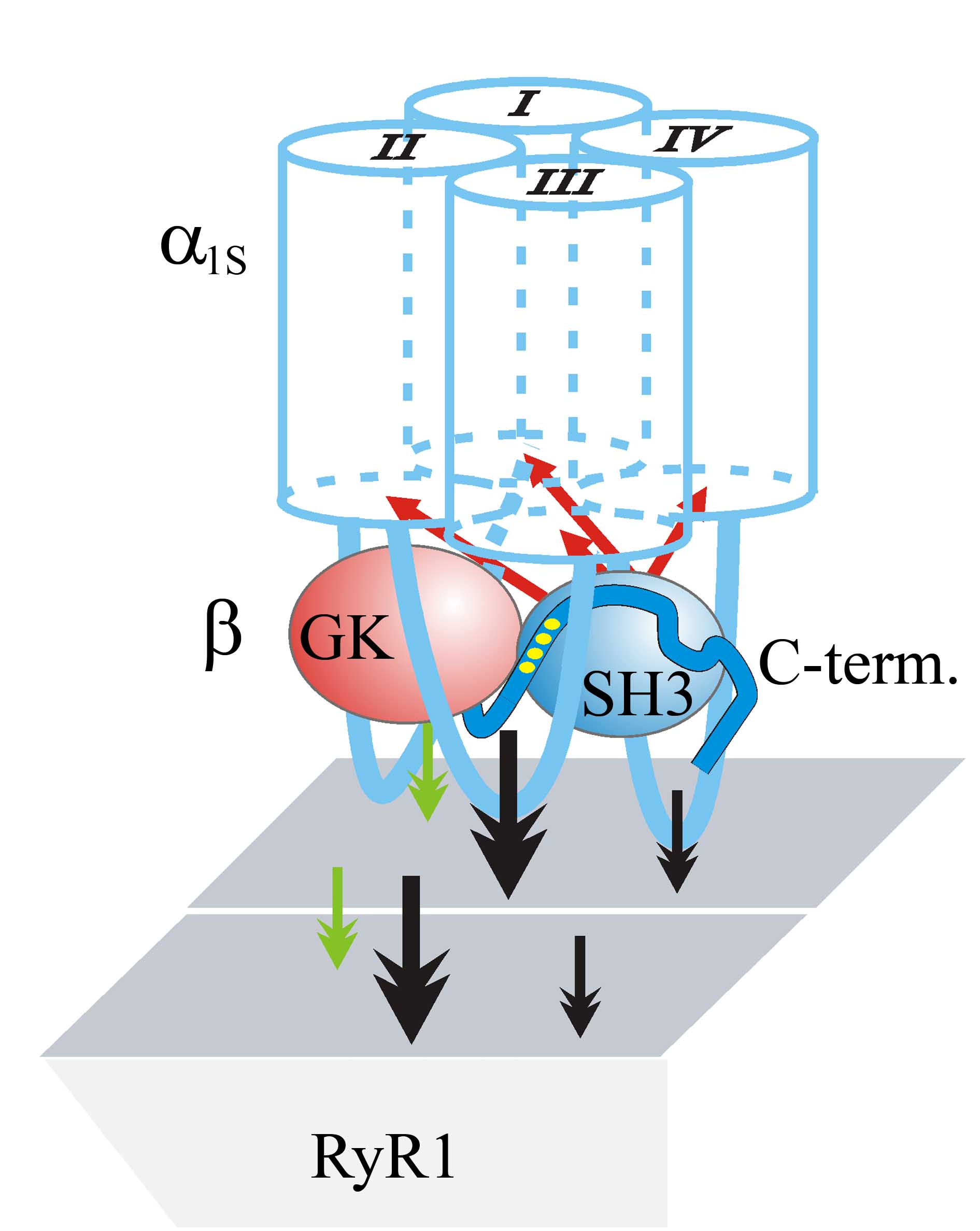
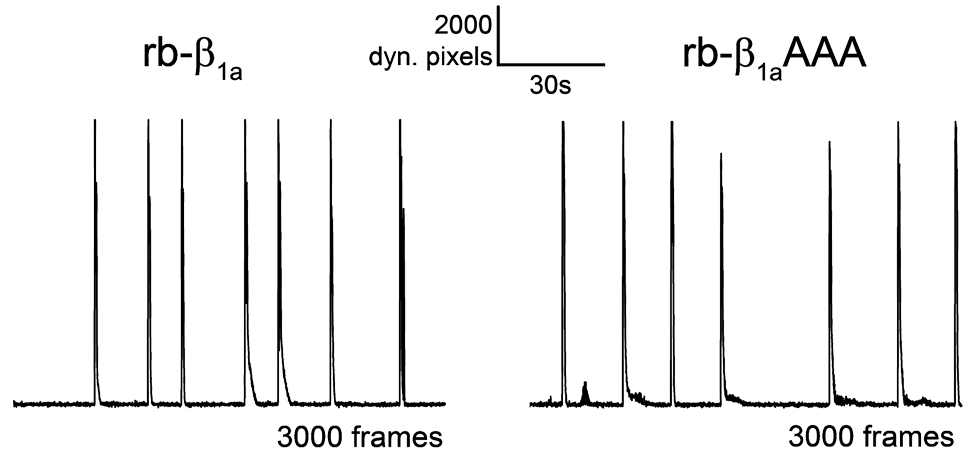
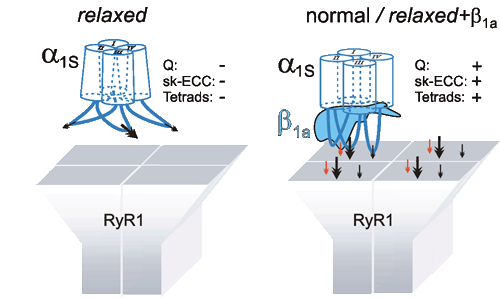
2013: In vitro expression of β1a/β3 chimeras in β1-null zebrafish relaxed myotubes revealed a pivotal role of the Src homology 3 (SH3) domain and C terminus of β1a in charge movement restoration. Furthermore, substitution of a P by A in the putative SH3-binding polyproline motif in the proximal C terminus of β1a (also of β2a and β4) fully obstructed α1S charge movement. Consequently, we postulate a model according to which β subunits, probably via the SH3-C-terminal polyproline interaction, adapt a discrete conformation required to modify the β1a conformation apt for voltage sensing in skeletal muscle. (cooperation with Franzini-Armstrong lab). Dayal et al., 2013, Proc Natl Acad Sci USA |
|
2010: In vivo and in vitro expression of the β1a-specific heptad repeat mutant in β1-null zebrafish relaxed revealed that the C-terminal heptad repeat motif in β1a is not the key determinant of EC coupling, as was postulated earlier by others. With this novel finding, the doors are reopened for in-depth structural-functional studies on the role of the β1a-subunit in skeletal muscle EC coupling. (cooperation with Franzini-Armstrong lab). Dayal et al., 2010, Cell Calcium |
|
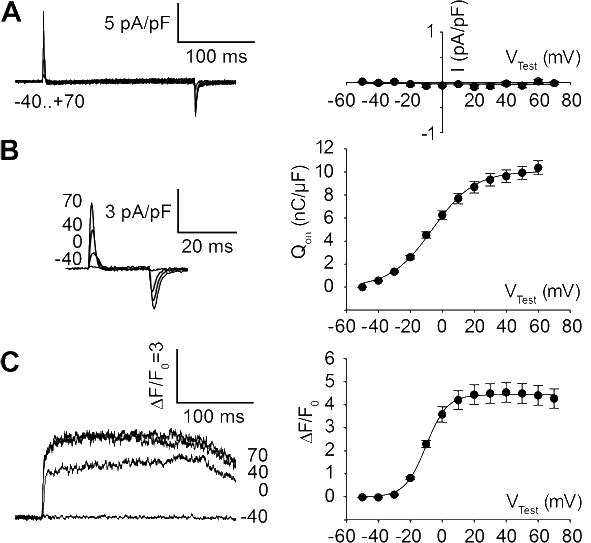
2010: Two distinct DHPR α1S subunits were detected in teleost fish muscle - differentially expressed in slow and fast muscle fibers. Both α1S isoforms did not conduct calcium ions, based on different conductance-blocking mutations, pointing to an evolutionary pressure towards non-calcium-conductivity in fish muscle. In Science Signaling: EDITORS' CHOICE: N. R. Gough, When a Channel Is Not a Channel. Sci. Signal. 3, ec92 (2010) Schredelseker et al., 2010, Proc Natl Acad Sci USA |
|
2009: Expression of different DHPR β-subunits in β1-null zebrafish relaxed revealed that triad targeting and charge movement restoration can be supported by every of the investigated β-subunits - but that proper tetrad formation and thus full restoration of EC coupling is an exclusive function of the β1a-subunit. (cooperation with Franzini-Armstrong lab). Schredelseker / Dayal et al., 2009, J. Biol Chem. |

|
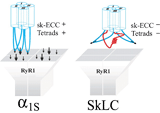
2005: Using the newly established model-system of a β1a-null zebrafish strain, the β1a subunit could be identified to be absolutely required for the formation of DHPR tetrads in skeletal muscle and thus for the DHPR-RyR1 protein-protein interaction. (cooperation with Flucher lab and Franzini-Armstrong lab) Schredelseker et al., 2005, Proc Natl Acad Sci USA |
|
2004: Expression of different DHPR α1 subunit chimeras in α1S-null myotubes and following analysis by immunocytochemistry and freeze-fracture electron microscopy revealed that the α1 II-III linker plays an important role in the formation of tetrads. The organization of DHPRs in tetrads is necessary but not sufficient for skeletal-type EC coupling. (cooperation with Flucher lab and Franzini-Armstrong lab) Takekura et al., 2004, Mol Biol Cell |
|
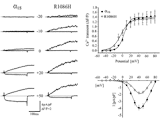
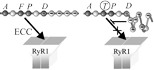
2004: Studying the DHPR α1S point mutation R1086H, associated malignant hyperthermia susceptibility, revealed, that this single mutation in the intracellular III-IV linker of the α1S enhances RyR1 sensitivity to activation by both endogenous (voltage sensor) and exogenous (caffeine) activators. (cooperation with Flucher lab and Dirksen lab) Weiss et al., 2004, Am J Physiol Cell Physiol |
|
2004: Analyzing the critical domain for bidirectional coupling in the DHPR α1S II-III linker on the single amino acid level revealed that the secondary structure of this region is an essential determinant for skeletal-type EC coupling. Kugler et al., 2004, J Biol Chem |

|
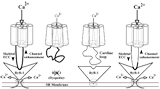
2000: Comparing targeting properties of the skeletal muscle CaV1.1 (α1S) with that of a neuronal isoform CaV2.1 (α1A) and appropriate chimeras revealed that the triad targeting signal of the skeletal muscle channel is located in the α1S C-terminus. (cooperation with Flucher lab) Flucher et al., 2000, J Cell Biol |
|
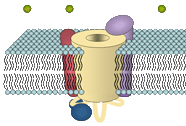
1999-2001: Several selected α1 chimeras were used to demonstrate that a critical domain of 45 amino acids in the α1S II-III linker is responsible for the bidirectional crosstalk of the voltage-sensing α1S with the sarcoplasmic Ca2+ release channel RyR1. (cooperation with Beam lab) Grabner et al., 1999, J Biol Chem Wilkens et al., 2001, Proc Natl Acad Sci USA |
|
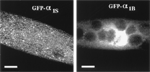
1998: Using N-terminal tagging of L-type and non-L-type α1 subunits with GFP we revealed that L-type channels are clustered into triads, whereas non-L-type channels seem to be diffusely distributed in the surface membrane. Important in later studies: GFP-tagged channels where shown to be fully functional in EC coupling. (cooperation with Beam lab) Grabner et al., 1998, Proc Natl Acad Sci USA |
|
|
EC coupling research in the Grabner lab - milestones
|
|
|
© 2011
|