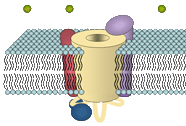
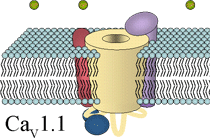
 CaV1.1 of the skeletal muscle surface membrane belongs to the group of high-voltage-activated L-type Ca2+ channels. L-type channels are highly sensitive to pharmacological active drugs, the so-called Ca2+ channel blockers phenylalkylamines (PAA), benzothiazepines (BTZ) and 1,4-dihydropyridines (DHP). The high-affinity binding of DHPs to CaV1.1 led to the synonym 'dihydropyridine receptor' (DHPR). The channel is composed of the central α1S subunit and of three additional (auxiliary) subunits, β1a, α2δ-1, and γ.
CaV1.1 of the skeletal muscle surface membrane belongs to the group of high-voltage-activated L-type Ca2+ channels. L-type channels are highly sensitive to pharmacological active drugs, the so-called Ca2+ channel blockers phenylalkylamines (PAA), benzothiazepines (BTZ) and 1,4-dihydropyridines (DHP). The high-affinity binding of DHPs to CaV1.1 led to the synonym 'dihydropyridine receptor' (DHPR). The channel is composed of the central α1S subunit and of three additional (auxiliary) subunits, β1a, α2δ-1, and γ.
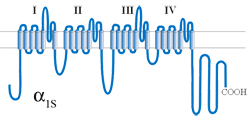 The central α1S subunit forms the channel pore and acts as the voltage sensor, enabling channel gating upon membrane depolarization. The α1 subunit is formed by 4 homologous repeats, each composed of 6 transmembrane helices that are linked by intracellular loops. The forth transmembrane helix in every repeat (S4) contains a number of positively charged residues responsible for the sensing of membrane depolarization and initiation of channel gating. The pore and the gate of the channel are lined by the fifth and sixth transmembrane helix of every repeat and their linking loops, the so called pore loop. Every pore loop contains one glutamate residue at a defined position, which, in the correctly folded channel, are in close proximity one to the other to form the Ca2+ selectivity filter.
The N- and C-termini as well as the intracellular loops linking the homologous repeats of the skeletal muscle α1S contain important functional domains, like e.g., the β-binding α-interaction domain (AID) in the I-II loop, the DHP-RyR-interaction domain (critical domain) in the II-III loop, and a triad-targeting domain in the C-terminus.
The central α1S subunit forms the channel pore and acts as the voltage sensor, enabling channel gating upon membrane depolarization. The α1 subunit is formed by 4 homologous repeats, each composed of 6 transmembrane helices that are linked by intracellular loops. The forth transmembrane helix in every repeat (S4) contains a number of positively charged residues responsible for the sensing of membrane depolarization and initiation of channel gating. The pore and the gate of the channel are lined by the fifth and sixth transmembrane helix of every repeat and their linking loops, the so called pore loop. Every pore loop contains one glutamate residue at a defined position, which, in the correctly folded channel, are in close proximity one to the other to form the Ca2+ selectivity filter.
The N- and C-termini as well as the intracellular loops linking the homologous repeats of the skeletal muscle α1S contain important functional domains, like e.g., the β-binding α-interaction domain (AID) in the I-II loop, the DHP-RyR-interaction domain (critical domain) in the II-III loop, and a triad-targeting domain in the C-terminus.
 The β1a subunit is bound intracellular to the α1S subunit. It is a ~ 60 kDa protein which shares structural similarities with the group of membrane anchored guanylate kinases (MAGUK) scaffolding proteins, although its function seems to be different and has been shown to play important roles in channel targeting, channel modulation and DHPR-RyR protein-protein interaction. The α2δ-1 subunit is a membrane spanning subunit with a large extracellular part, which is highly glycosylated. It is formed by two peptides α2 and δ, which are tightly linked by disulfide-bonds and thus virtually act as one subunit. The α2δ-1 has been shown to have remarkably effects on the modulation of channel kinetics. The membranous γ subunit is a unique accessory subunit of the skeletal DHPR and seems to play only a minor role for the channel function, and thus a γ k.o. mouse model shows undisturbed EC coupling.
The β1a subunit is bound intracellular to the α1S subunit. It is a ~ 60 kDa protein which shares structural similarities with the group of membrane anchored guanylate kinases (MAGUK) scaffolding proteins, although its function seems to be different and has been shown to play important roles in channel targeting, channel modulation and DHPR-RyR protein-protein interaction. The α2δ-1 subunit is a membrane spanning subunit with a large extracellular part, which is highly glycosylated. It is formed by two peptides α2 and δ, which are tightly linked by disulfide-bonds and thus virtually act as one subunit. The α2δ-1 has been shown to have remarkably effects on the modulation of channel kinetics. The membranous γ subunit is a unique accessory subunit of the skeletal DHPR and seems to play only a minor role for the channel function, and thus a γ k.o. mouse model shows undisturbed EC coupling.