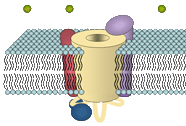
 Vertebrate skeletal muscles are formed by large syncytial cells, the myotubes, which are innervated by excitatory nerves via the motor endplate. Contraction of these myotubes is induced by neuronal excitation and further propagation of an action potential along the surface membrane. Inside multiple invaginations of the surface membrane (the T-tubules) the plasmalemma is closely juxtaposed to the membranes of the sarcoplasmic reticulum (SR) in a sandwich-like alignment; a formation called the skeletal muscle triad. The close proximity of the membranes in the triadic junction allows interaction of two distinct Ca2+ channels that mediate the transduction from membrane depolarization to the contraction of the cell, a process called excitation-contraction (EC) coupling.
Vertebrate skeletal muscles are formed by large syncytial cells, the myotubes, which are innervated by excitatory nerves via the motor endplate. Contraction of these myotubes is induced by neuronal excitation and further propagation of an action potential along the surface membrane. Inside multiple invaginations of the surface membrane (the T-tubules) the plasmalemma is closely juxtaposed to the membranes of the sarcoplasmic reticulum (SR) in a sandwich-like alignment; a formation called the skeletal muscle triad. The close proximity of the membranes in the triadic junction allows interaction of two distinct Ca2+ channels that mediate the transduction from membrane depolarization to the contraction of the cell, a process called excitation-contraction (EC) coupling.
Upon membrane excitation a voltage-dependant Ca2+ channel, the plasmalemmal, multi-subunit dihydropyridine receptor (DHPR), acts as the voltage sensor and triggers the Ca2+ release from intracellular Ca2+ stores (sarcoplasmic reticulum; SR) through a sarcoplasmic Ca2+ release channel, the ryanodine receptor (RyR). In consequence to RyR opening, Ca2+, released to the cytoplasm, can bind to troponin inside the actin filament to activate the "muscle ratched mechanism" and thus induce muscle contraction.
In cardiac myocytes the action-potential induced opening of the DHPR results in a considerable influx of Ca2+, which in turn is the crucial trigger for the opening of the Ca2+-sensitive RyR2. Not so in skeletal myofibers: Here the transduction of the signal from the DHPR to the RyR is independent of the influx of extracellular Ca2+. Instead, the skeletal-type DHPR exclusively acts as a voltage sensor. Its depolarization-induced activation is thought to induce a conformational change, which in turn is responsible to open the RyR via direct protein-protein interaction. However, a small influx of Ca2+ through the DHPR can be observed in mammalian skeletal muscle, which is so far not fully understood.
A precise targeting is indispensable for the interaction of the two channels. In skeletal, as well as in cardiac muscle cells DHPRs are targeted into the triadic plasmamembrane, where the channels aggregate in clusters located oppositely to clusters of RyRs in the SR membrane. For protein-protein interaction of the channels in skeletal muscle it is further required that four DHPRs congregate in a square formation opposite the four domains of the RyR; a constellation known as skeletal muscle tetrad.